PHYSICO-CHEMICAL
PROPERTIES OF (±) IQB-9302 HYDROCLHORIDE
ASPECT
White crystalline powder, inodourous,
bitter taste that rapidly diseappears because of anesthesia of oral mucosae.
NAME AND STRUCTURE
-
Chemical Name: dl -1-(methylcyclopropyl)-N-(2,6-dimethylphenyl)-2-
piperidinecarboxamide; dl-1-(methylcyclopropyl)-2',6'-pipecoloxylidide.
>
-
Chemical structure:
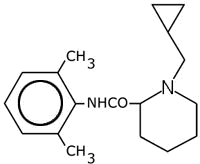
-
Molecular formula and molecular weight:
C18H26N2O; mol.weight: 286.42
-
as hydrochloride: C18H26N2O.HCl;
mol.weight: 322.88
CAS-REGISTRY NUMBER
IQB-9302 base racemic [166181-63-1]
IQB-9302 hydrochloride racemic [166546-89-0]
SYNTHESIS (scheme)
IQB-9302 is obtained in good yield
from 2,6-dibromohexanoyl-2,6-dimethylaniline and aminomethylcyclopropane,
heating the mixture for 16 hours in an adecuate organic solvent. IQB-9302
is obtained as free base by acidic extraction and washing. Yield 70%.
Hydrochloride salt of IQB-9302 is
obtained by reaction of the free base with HCl in isopropanol and recrystallization.
SOLUBILITY
Soluble in water (88,4 mg/mL),
DMSO and methanol. Soluble in hot ethanol. Insoluble in acetone, chloroform,
ether, methylene chloride, etc.
MELTING POINT
263.4º C (as HCl) (decomp.)
THERMAL ANALYSIS
Thermal behaviour of samples of IQB-9302
was studied with a Thermogalen microscope fitted with the Kofler stage.
Samples were studied between 30 and 300ºC at a heating rate of 2ºC/min.
At room temperature the test material was constituted by needle-shaped
crystals. With polarized light the sample appeared as coloured particles.
In the range of 172-210ºC recrystallization by sublimation was detected.
The new crystalline particles showed laminar aspect and were coloured even
without polarised light.
Three batches of IQB-9302 were analyzed.
All the samples melted in the interval 245-254ºC.
DIFFERENTIAL SCANNING CALORIMETRY
Thermodynamic and calorimetric properties
of IQB-9302 have been assessed by
thermographic analysis performed
on a Mettler TA3000 System. Neither hydrates or solvates, nor polymorphs
are seen. Chemical purity of IQB-9302 ranged between 99 and 100%. On the
base of DSC data, lot 9454/R003 has been selected
as standard for
chemical analysis (see here
purity
of other batches).
THERMOGRAVIMETRY
Thermogravimetric curves were obtained
using a Mettler TA 3000 thermogravimeter analyzer (TG50). Samples were
placed into an alumina crucible and heated at a rate of 10º/min in
the range of 30 to 300ºC. Data demonstrated the absence of solvates.
Decomposition process occurs in one step. Three batches gave identical
TG curves
ULTRAVIOLET SPECTRUM
Aqueous solution
100 mg/ml in distilled water lmax
= 262.4 nm Abs= 0.120
Acetonitrile/phosphate
buffer 500 mg/ml 0.1 M, pH 5 (40:60) lmax
= 262.6 Abs = 0.646.
No shifts of lmax
were seen when IQB-9302 was dissolved in either 01N HCl or 0,1. N NaOH.
UV scans of three batches were identical, a proof that there is not batch-to-batch
variability
INFRA-RED SPECTRUM
Principal peaks
at wavenumbers 3104, 2938 , 2606 and 1670 cm-1 (KBr disk) .
The IR spectra of three batches were practically identical thus demonstrating
the absence of batch-to-batch variability
MNR SPECTRUM
1H-RMN.
(DMSO-d6) d in ppm:
10.62 (s,1H); 9.84 (s, 1H); 7.08
(m, 3H); 4.27 (m,1H); 3.10 (m, 2H), 2.14 (m, 1H); 2.06 (m, 6H), 1.90-1.00
(m, 6H); 0.68 (t, 2H); 0.36 (t, 2H)
X-RAY DIFFRACTION SPECTRUM
The powder X-Ray diffraction spectra
of several samples of IQB-9302.HCl were recorded using an automated Philips
X-Pert X Ray diffractometer. Samples were irradiated with monochromatized
CuK alfa radiation and analyses were caried out between 2 theta
angles
of 5 and 40º.
Characteristic diffraction lines
are seen at angle values of 8.39, 11.640, 16.605, 17.730, 19.175, 21.455,
23.565, 25.390, 26.795 and 28.830. No differences in the X-ray diffraction
spectra
of five different batches of IQB-9302.HCl were observed thus confirming
the absence of polymorphs.
HIGH PRESSURE LIQUID CHROMATOGRAPHY
Column. Hypersil ODS 5
mm (12 cm, f =
4.6 mm)
Eluent: Acetonitrile/phosphate buffer
0.1 M, pH 3.5 (30:70)
Flow: 1.0 ml/min
Detector: UV at
l = 262 nm
Volume of injection: 20 ml
Sample concentration: 500 mg/ml
in eluent
Rt = 4.3
Purity (of batch
940531) = 99.3% (See
here
purity of other batches)
THIN LAYER CHROMATOGRAPHY
Plate: Kieselgel Merck 60F 354
Solvents: Diisopropyl ether, Rf
=
0
Ethanol, Rf=
0.76
Acetone/methylene
chloride (95:5), Rf
= 0
DISSOCIATION CONSTANT
pKa: 7.99 ± 0.006
PARTITION COEFFICIENT
n-heptane/buffer pH 7.4 = 68
n-heptane/n-octanol =86
GLPs: Most of analytical
tests were carried out according to EMEA guidelines at the Department of
Galenics (Faculty of Pharmacy, University of Madrid) and at LEBSA Study
Director: Prof. M.D. Veiga, Ph.D. Pharmacy
LEBSA Scientists: Dr. J.M. Espinós,
Ph.D. Chemistry
Involved Scientists: M. Merino,
B.S. Faculty of Pharmacy
Anna Pons, B.S.
QAU LEBSA: Mª Luisa Espinós,
B.S,
QAU IQB: A. Soria. B.S
Report: Phsycochemical
Properties of IQB-9302.HCl |
STABILITY OF RAW MATERIAL
Samples of IQB-9302 (as HCl) have
been maintained for 4 years at room temperature under normal light and
humidity with no apparent loss of activity. Additionally, the following
tests have been done in order to accelerate degradation of the raw material:
1. In solution
A1 - 1% solution in water
24 h at room temperature (shaking): Unchanged (100%)
R1 - 1% solution in water
24 h under reflux. Practically unchanged (99.13%)
A2 - 1% solution in HCl 0.1N
24 h at room temperature (shaking).Unchanged (100%)
R2 - 1% solution in HCl 0.1N
24 h under reflux. Practically unchanged (99.13%)
A3 - 1% solution in NaOH 0.1N
24 h at room temperature (shaking).Unchanged (100%)
R3 - 1% solution in NaOH 0.1N
24 h under reflux.Unchanged (100%)
2. Stability
of the solid
A1 - Under UV light:Unchanged (100%)
A2 - At 100º C for 2 months:
small degradation 96.77% IQB-9302.HCl
A3 - Melted state (24 h): 11.01%
IQB-9302.HCl; 67.37% 2,6-dimethylanilide.
DRUG MASTER FILE
A Drug
Master File covering chemical synthesis, analytical methodology, impurities
and other aspects of IQB-9302.HCl has been filled by LEBSA, manufacturer
of the compound on the behalf of Laboratorios INIBSA
GMPs: Drug Master File has
been filled according to FDA regulations by LEBSA, manufacturer of IQB-9302.HCl
Study Director: Dr. J.M. Espinós,
Ph.D. Chemistry
Involved Scientists:Anna Pons, B.Sc.
Silvia Dieguez, B.Sc.
QAU Lebsa: Mª Luisa Espinós,
B.Sc.
QAU IQB: A. Soria. B.S. |
|


